 |
|
| Browse | Ask | Answer | Search | Join/Login |
|
|
||||
|
About lewis structure of SI6
Lewis structure of SI6 is this as you know :
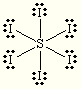 My question is , how come that S , can be connected to 6 Iodine molecules, (which means S, is connected to 12 electrons instead of 8 ), while S needs only two electrons to reach an octet? |
||||
|
||||
|
I would say surely DrBob :) You perhaps know (or will learn maybe soon) that electron shells consist of orbitals, the s, p, d and f orbitals. The first electron shell contains only s orbital, the second shell contains s and p orbitals, the third, s, p and d while the fourth and above contain all of s, p, d and f. Since the last electrons of sulfur are in the third shell, you have the s, p and d orbitals available. S can contain 2 electrons, p can contain 6 electrons and d can contain 10 electrons, for a total of 18 electrons! |
||||
Not your question?
Ask your question
View similar questions
| Question Tools | Search this Question |
Add your answer here.
Check out some similar questions!
Lewis structure
[ 3 Answers ]
The central atom in a molecule is the one with the highest electron affinity
Draw lewis structure
[ 10 Answers ]
Draw the Lewis structures for each of the following ions or molecules. For each, give (I) the Molecular shape, (ii) the electron pair geometry at the central atom, and (iii) the hybridization of the central Atom. (a) IO4 - (b) ClF5 (c) IOCl3 (d) CO2 (e) NHF2
Lewis Structure
[ 1 Answers ]
What is the lewis dot structure for IO-2
Lewis Structure
[ 1 Answers ]
What is the lewis dot structure for IO-2
Lewis Structure
[ 3 Answers ]
What does the Lewis structure of ClF4- look like?
View more questions Search
|




