The ideal gas law is
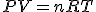
where P=pressure, V = volume, n = number of moles (what you're trying to solve for), R is given to you, and T is the
absolute temperature (in Kelvins). You need to find P, V, and T in order to fine n.
The compression ratio is 16 to 1. You assume the 1 is atmospheric pressure. Solve for the pressure.

Y
You should be able to find V and T at the maximum compression from table 5.6.
i need more help on this. how do i find V and T from table 5.6. and how do i find out how many moles of gas are present in one cylinder during a cycle. please can you explain it to me further.
The ratio mass/power is important because power is required to move the mass (especially up hill and down hill. I'm sure you can think of other reasons.
Since this is a volume, I assume that this is from the position of the piston in the cylinder. You could calculate that if you had a knowledge of the diameter of the cylinder and the position of the piston in the cylinder.
You calculated the number of moles in #1.

multiply that by the number of moles and you should have the answer, if I understand the question correctly.
what is the number of moles and what do i multiply it by, i am really confused about all this. can you please tell me.
The table shows the work done on the gas. This work results in increased internal energy. Just calculate it.
how would i do this, would you be able to show me please. i cannot find the work one on the gas which you say is provided on the table.
I don't see the diagram, so I might not get this exactly. Basically, you have to calculate the work done on the system and the work done by the system. Subtract them to get the theoretical efficiency.
This is similar to #6, except you have information on actual output. I don't remember seeing anything in your data about the fuel, though chemically this could be used to calculate input enthalpy.
Hope this helps. I really can't do all the problems for you. That's against the rules.

