The mass of 1 cubic meter (?) of air is 129 kg. The mass of air is proportional to its volume.
The "combined gas law" (combining Boyle's, Charles, and Gay-Lussac's laws) is:
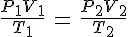
at STP,
P1 = 1 atm = 101.32500 KPa
P2 = 96 KPa
V1 is unknown for the volume specified.
V2 is given, 8 x 6 x 4 m = 192 m^3
T1 at STP is 273.15 Kelvins (0 °C).
T2 is 293.15 Kelvins (20 °C)
Solve the equation for V1 (the volume at STP). Set up a ratio equation wherein 1 cubic meter is to 129 Kg as V2 cubic meters is to the unknown weight. Solve for the unknown weight.
I've set it up for you. You can do the math.
This should have been put under Chemistry or possibly Physics.

