 |
|
|
 |
New Member
|
|
Apr 14, 2009, 03:13 PM
|
|
|
Orbital notation
Does anyone know how to do orbital notation? I'm so :confused:
|
|
|
 |
Senior Member
|
|
Apr 14, 2009, 03:21 PM
|
|
|
Isn't that math? IF you haven't posted you question in the math thread, then you may want to do that. Sorry I'm not sure what it is. Good luck eh.XD
|
|
|
 |
Uber Member
|
|
Apr 14, 2009, 03:23 PM
|
|
|
Do you mean electron configuration - what are you having trouble with?
|
|
|
 |
New Member
|
|
Apr 14, 2009, 03:31 PM
|
|
|
 Originally Posted by Capuchin

Do you mean electron configuration - what are you having trouble with?
Yeah - well I have three boxes one says electronic configuration, 2nd says orbital notation, and 3rd says noble gas notation. I have the elctronic configuration filled out but I don't understand how to fill out the orbital notation.
|
|
|
 |
Uber Member
|
|
Apr 14, 2009, 03:35 PM
|
|
|
For orbital notation you need to describe the actual orbitals and how many electrons are in them, i suppose, for example
 etc.
Have you been tauight how to do that?
|
|
|
 |
New Member
|
|
Apr 14, 2009, 03:40 PM
|
|
|
 Originally Posted by Capuchin

For orbital notation you need to describe the actual orbitals and how many electrons are in them, i suppose, for example
 etc.
Have you been tauight how to do that?
yeah I'm just a little confused on how I'm suppose to describe them etc because he explained it really fast
|
|
|
 |
Uber Member
|
|
Apr 14, 2009, 03:51 PM
|
|
|
Well, the number in front is the electron configuration level, so 1s2 is the inner most ring, 2s2 and 2p6 make up the second ring etc. the letter is just the type of orbital, they have different energies and you really just have to remember the letters. The number after the letter (and superscript) is the number of electrons in this level.
You should commit to memory how to construct a table like the folowing:

This describes the order that the orbitals fill up (approximately). You basically just keep filling up the levels (following the arrows) until you run out electrons. Does that help, do you still have questions?
|
|
|
 |
New Member
|
|
Apr 14, 2009, 03:54 PM
|
|
|
 Originally Posted by Capuchin

Well, the number in front is the electron configuration level, so 1s2 is the inner most ring, 2s2 and 2p6 make up the second ring etc. the letter is just the type of orbital, they have different energies and you really just have to remember the letters. The number after the letter (and superscript) is the number of electrons in this level.
You should commit to memory how to construct a table like the folowing:

This describes the order that the orbitals fill up (approximately). You basically just keep filling up the levels (following the arrows) until you run out electrons. Does that help, do you still haev questions?
That helps a lot! Thank you so much :D
|
|
|
 |
New Member
|
|
Aug 23, 2009, 05:45 AM
|
|
|
 etc.
what do you call this?(i have the same problem with sunni_hubbard:D)
|
|
|
 |
New Member
|
|
Aug 23, 2009, 05:47 AM
|
|
|
 Originally Posted by Capuchin

 etc.
what do you call this?(i have the same problem with sunni_hubbard:D)
|
|
|
 |
Uber Member
|
|
Aug 24, 2009, 03:00 AM
|
|
|
Orbital notation, I guess. I'm not sure of the exact name.
|
|
|
 |
Uber Member
|
|
Aug 24, 2009, 11:54 AM
|
|
|
Perhaps detailed electronic configuration... well in the OP's question, it was orbital notation.
I have another way to look at that, it's more 'linear'.
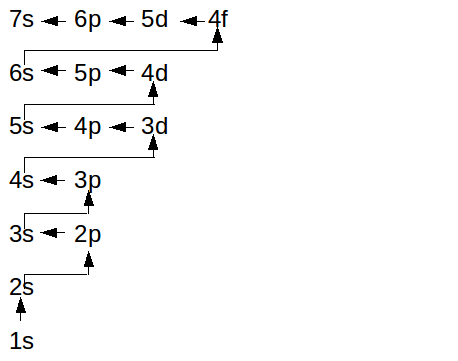
|
|
|
 |
New Member
|
|
Nov 16, 2009, 04:03 PM
|
|
|
This is amazzzinggg!! Thank you!!
|
|
|
 |
Junior Member
|
|
Nov 29, 2009, 05:40 AM
|
|
|
Sorry Sunni Hubbard neither do I but here is a little more help with electron configuration
The coefficient tells what electron shell it is and also tells what row it is
The letter tells what subshell it is or column
The superscript tells how many electrons it holds
Back to letters, the letter "columns" are for the most part s,p,d and f in that order
S holds 2 p 6 d 10 and f 14
To create a configuration, first find how many valence electrons, shells, and electrons it has
Then mark the columns and last shell
At the top the first row holds up to s 2nd up to p 3rd and farther f
Now keep filling up the shells and count the electrons you used
You may need to tweek what you did but once you have the number of electrons it is finished
|
|
|
 |
Junior Member
|
|
Nov 29, 2009, 05:41 AM
|
|
|
Sorry third to d
|
|
|
 |
Junior Member
|
|
Nov 29, 2009, 05:44 AM
|
|
|
Do you mean creating a diagram of the atom focusing on the electrons by drawing circles as shells and putting in circles as electrons, Ive done that before
|
|
|
| Question Tools |
Search this Question |
|
|
|
Check out some similar questions!
Hybridized Orbital
[ 1 Answers ]
What is the percent s character in an sp3 hybridized orbital?
A) 25%
B) 33%
C) 50%
D) 67%
E) 75%
View more questions
Search
|











