 |
|
| Browse | Ask | Answer | Search | Join/Login |
|
|
||||
|
pressure difference
Lets say if I have a test tube (diameter 3cm and height 30cm) close with a stop rubber. If air was flow into the test tube from bottom of the tube with flow rate around 20L/min, to mix the reactant and after a few sec, the rubber was push out.. in general I understand that the pressure inside the test tube is higher than atmospheric pressure. But I can't find any mathematical solution that relate flow rate and pressure to proof that the pressure inside the test tube is higher than outside. Can anyone explain it to me.
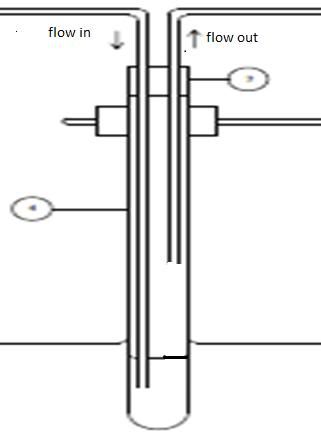 inlet air flow rate = 20L/min outlet air flowrate = 38 L/min density of liquid = 1007.56 kg/m3 tubing inlet diameter = 1 cm tubing outlet diameter = 0.45 cm inlet length of tubing = 30 cm outlet length of tubing = 40 cm volume of liquid = 100 mL air temperature = 25 C liquid temperature = 60 C |
||||
|
||||
|
I'm confused because the outlet air flow rate is higher than the inlet rate. That should result in the opposite behavior! They must be measured at different pressures?
Anyway, in general if you want to compute the change in pressure after a certain amount of time, you need to compute the net amount of air that flows in or out during that time. With the numbers you've provided, for example, if I want to know the net air in or out after 1 minute, I can see that 20L went in, but 38L came out, so a net of 18L came OUT of the test tube. If the pressure of the air going in is different than the pressure of the air going out, you're better off using this equation: PV/T(before) = PV/T(after) It simply states that P x V / T (pressure times volume divided by temperature[in Kelvin - not Celsius!]) stays the same for a given amount of gas. If you shrink the volume but keep the temperature the same, the pressure goes up accordingly. If you cool the temperature but keep the volume the same, the pressure will drop. Etc. etc. So in this case, in one minute you're putting in 20L of gas at a temperature of 298K (at some pressure which you haven't specified). That 20L of gas has to squeeze into the available volume in the test tube. Keep in mind that the test tube also has liquid in it, and you can usually assume that the liquid is virtually incompressible. In other words, the available volume for the gas is equal to the entire volume of the test tube minus the volume of the liquid. So in this case the test tube volume is about 212 cm^3, and the liquid volume is 100 cm^3, so the net volume available for the gas is about 112 cm^3. So you can plug in the numbers (including the unspecified inlet pressure) and solve for the new pressure of the inlet air after it's squeezed into a volume of 112 cm^3 and heated to 333K. This pressure will ADD to the pressure that existed inside the test tube before. In other words, if the initial pressure in the test tube was 1 atm, and you find that the 20L of gas you pumped in will end up at a pressure of 14.2 atm, the total pressure inside the test tube would be 15.2 atm after 1 minute (except we haven't yet accounted for the gas flowing out through the outlet so that will drop the pressure back down somewhat). Meanwhile, during that same minute 38 L of air flowed out of the test tube, measured at some other pressure that you didn't specify (presumably flowing out at a temperature of 333K). So now, knowing the pressure, volume (38L), and temperature (333K) outside of the test tube, you can use the same formula to calculate how much pressure that gas must have been under when it was crammed inside the 112 cm^3 test tube. That's how much the pressure will have been REDUCED inside the test tube after 1 minute. So now just put it all together. Let's say the initial pressure was 1 atm, the gas that went in would end up at a pressure of 14.2 atm, and the gas that came out of the outlet would have been at 12.1 atm inside the test tube (again, I'm just making up these numbers since I don't know the pressures at the flow meters on the inlet and outlet), that's a net result of 1 + 14.2 - 12.1 = 3.1 atm of pressure inside the test tube after 1 min. One final thing: It seems rather unlikely to me that the flow rate in and out remain constant unless you're actively controlling the flow rate the whole time (with mass flow controllers or whatever). Is this the case? Otherwise, if your inlet flow comes from a tank with a regulator or a diaphragm pump, etc. the flow rate may start at 20 L/min, but as the pressure in the test tube rises, that rate will drop. Meanwhile, as the pressure in the test tube rises, the outlet flow rate will increase. |
||||
|
||||
|
This looks totally screwed up to me. I flunked my Intro to Chemical Engineering course at MIT, but I still remember that Input equals Output plus Accumulation. So, if you bring in gas at 20 L/min and remove it at 38 L/min you won't blow the top. In fact you will create a VACUUM in a couple of seconds.
With an outlet of smaller diameter than the inlet you will have a natural net inflow. Are you sucking out the gas? And, my God, at these flow rates you will blow the liquid right out of the tube! But, to answer your original question, if more gas comes into the tube (flow in > flow out) the pressure must rise. |
||||
| Question Tools | Search this Question |
Add your answer here.
Check out some similar questions!
My 40/60 3/4hp shallow well jet pump will run continuously and will not build up air pressure in the bladder. The bladder will hold pressure though. I have filled it with air and when I turn on the water it loses its pressure and when the pump kicks on it just keeps running without building the...
What is the difference between a pump tank and a pressure tank? Thanks Mike
When the yellow jacket brand gauges are put on a 3 ton Tempstar heat pump what should the correct suction pressure and head pressure be. I suspect it to be overcharged in freon. Because it is freezing the compressor, accumulator tank and all the lines soild ice.
Hi, I just moved into an older house and I'm having some issues with the hot water and the cold water having different water pressure. It's like this through out the house. The cold water is virtually non-exsistant and the hot water is out of control. It's like this from the kitchen sink to...
View more questions Search
|




