 |
|
| Browse | Ask | Answer | Search | Join/Login |
|
|
||||
|
Is the MAXIMUM density of water a constant?
Please bear with me. I'm sorry if I'm hard to follow.
I know that under 1 standard atmosphere of pressure, water has the following properties: Freezing/melting point of 0 Celsius Boiling/condensing point of 100 Celsius Maximum density of 1.0 g/cm^3 achieved at 4 Celsius Here's my question: If water were under 0.5 standard atmospheres of pressure or 2 or 3 standard atmospheres of pressure, what numbers would correspond with the above properties and most importantly, would the Maximum density still be 1.0 g/cm^3 and would that still be achieved in the liquid phase? I do NOT need to know about extreme environments like the high pressures at the bottom of the ocean, or like the vacuum of space. That data is irrelevant to me because I'm only considering pressures at which breathable atmospheres would exist. Thank you very much and I would love a formula and numbers or a link to a table regarding the all-important MAXIMUM density question: Is the MAXIMUM density that water achieves always 1.0 g/cm^3 under "air-like" conditions that would exist in a range of atmospheric pressures? (not under water) |
||||
|
||||
|
No, maximum density depends on pressure, and will be higher at higher pressures (pretty obviously.. ). The temperature at which maximum density occurs also decreases at higher pressure. Something like this: 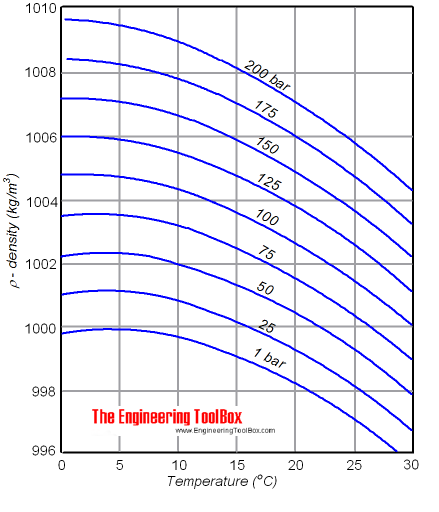 PS. I don't know why you think that bottom of the ocean or outer space is irrelevant to you - the same laws work over the entire ranges of pressures and temperatures. |
||||
|
||||
|
Okay, I'm actually uneducated in physics, so I'm not quite sure of a couple of things. 1 bar is the same as 1 standard atmosphere, right?
Also, there is a reason that I'm not interested in extreme conditions. I understand that the same laws hold true, but for this case I specifically need to know about the range of pressures from about 1/3 or 1/2 standard atmosphere to 3 or 5 standard atmospheres. I appreciate the graph. However, its spread is so vast. Is there some place I could find more confined information with details across the above mentioned range for freezing, boiling and maximum density? |
||||
| Question Tools | Search this Question |
Add your answer here.
Check out some similar questions!
When we bought our house, it came with a decommissioned water softener in the laundry room. The pipes behind it (I'm assuming in and out) were capped off with plastic plugs that toggle back and forth. If you're familiar with Dr. Dolittle, I'd call it a pushmi-pullyu setup. (sorry, I'm an admin, not...
Hi. I'm having difficulty reaching 50psi in my water pump. It reaches 40psi and just continues to run. I've adjusted the air pressure in the pressure tank, but that didn't work. The tank had no air pressure when I checked it earlier today. I followed the procedure listed on the air tank to get...
Maybe this belongs under Chemistry, but if water cannot get hotter than 212 degrees at sea level before boiling, why do some recipes call for faster (rolling?) boils rather than a simmering boil? Sometimes things other than water are heated/boiled, but I believe the same underlying principle...
View more questions Search
|




